Catatan

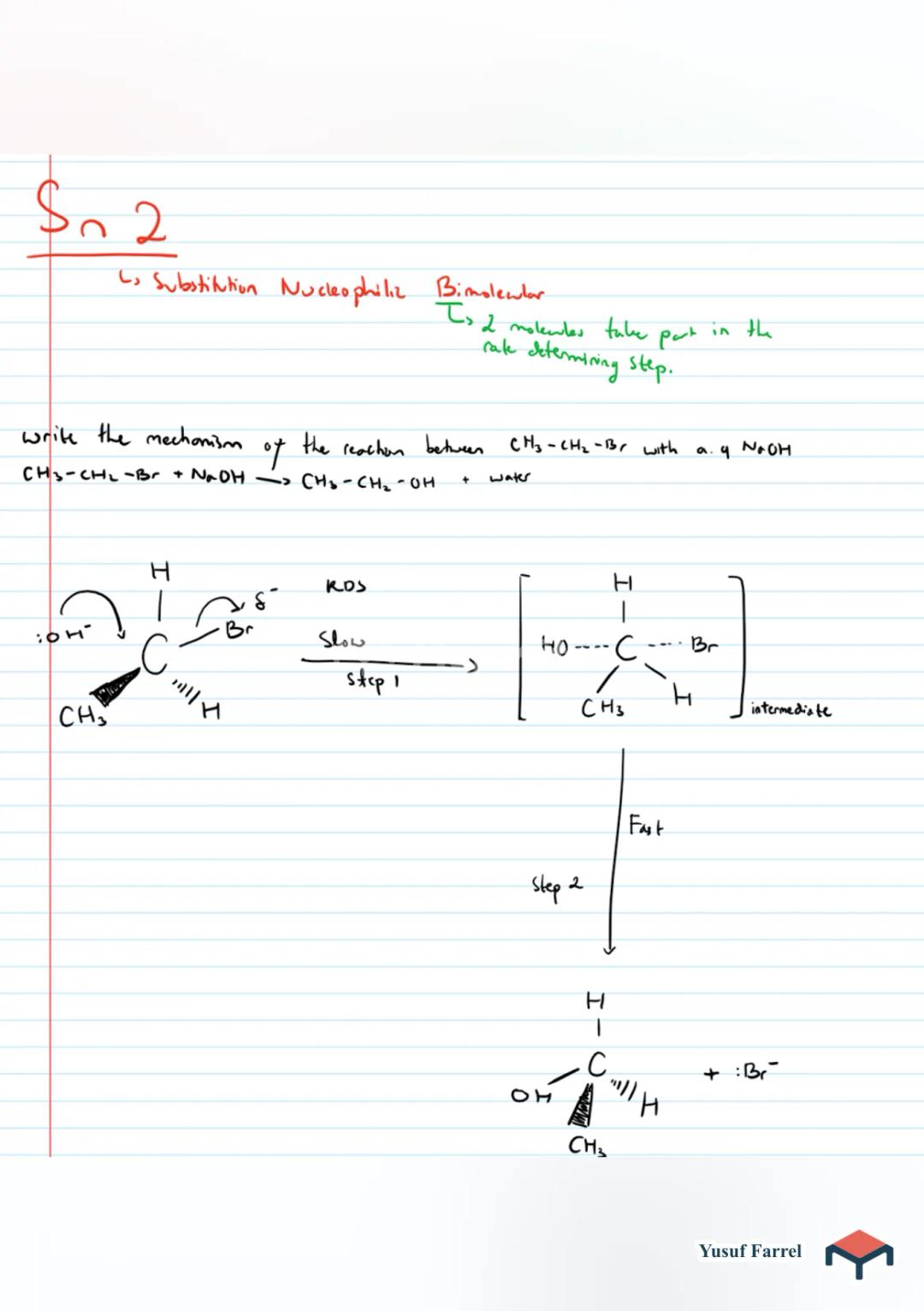
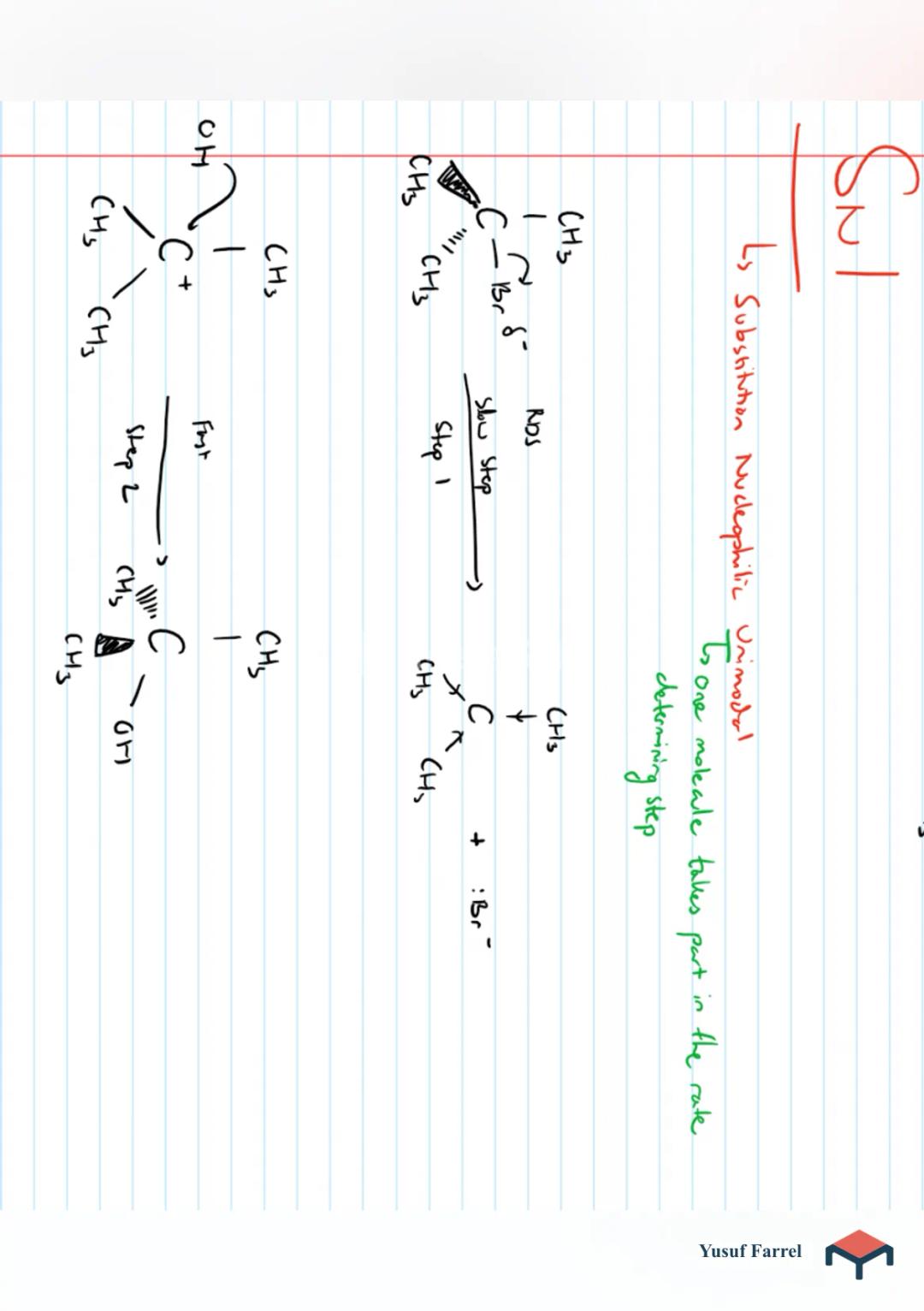
Organic Chemistry - Halogenoalkanes
Rangkuman by MejaKitty
P increases with molewler Size due to increased B.Soluble in organe solvents but insoluble di waterCH3 - CH (B,Is dipole is incred inc x bond and it becomes polar. thus to attack by nucleophile ) open-

Dibagikan oleh
Yusuf Farrel
Sebuah catatan agak rapih oleh Farrel, semoga membantu :)
Kimia
SMA / SMK
Success